ilooda
develops products that meet international safety standards so that healthcare providers who choose our products can safely carry out their services and emergency patients can receive safe treatment. We comply with all Korean and overseas laws and international agreements related to the environment and healthy and safety. We also carry out local and overseas certification and maintenance services through licensing and quality management for medical equipment.






Global Reliability
With our licensing team and representatives who identify key variables, we aim to provide the highest quality products by securing entry to the global market. To this end, we have obtained global licenses and complied with medical equipment product quality systems in 48 countries.

From development planning to Life Cycle workforce management and process establishment.
Innovation
Does Not Emerge From a Bubble.
ilooda’s drive to innovate to help build healthy and beautiful lives is
based on powerful technology rooted in robust clinical validations
and our licensing strategy, which we can demonstrated
through our trusted network of medical professionals.
Clinical and medical staff
verification system PROCESS
symptoms
Hospital
Medical Device Demonstration Cluster
evaluation
documents

protocol

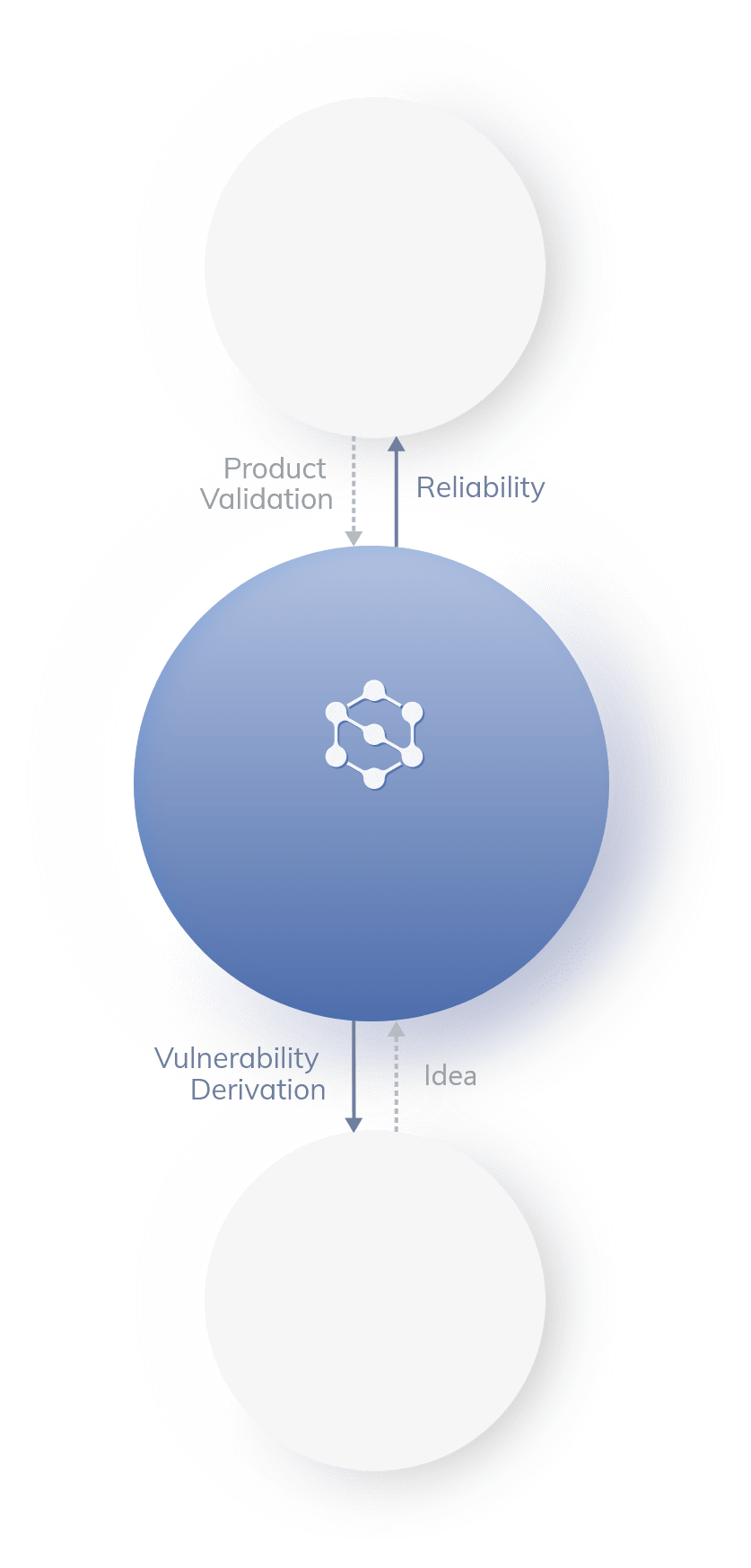
(Medical device
development)
(Intermediate
clinical trials)
We are building a clinical network with around
67 key doctors
from 52 countries.