ilooda at a Glance
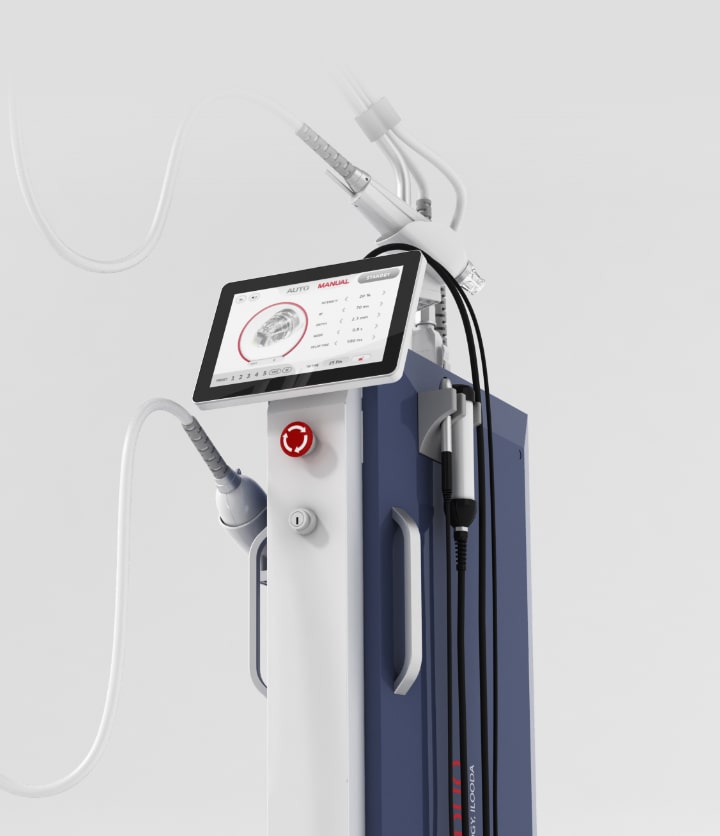

In 2006, a group of innovators from among Korea's top medical device researchers came together to carve a path through the barren land of Korean medical equipment manufacturing technology, founding ‘ilooda’ for the purpose of carrying out 'true research.'
Over the 15 years since ilooda’s founding, we have strived to develop honest and unpretentious medical equipment based on the uncompromising spirit of pursuing technological perfection.
good health, and
beautiful dreams.


Head Office relocation / Opening the Anyang branch office
Academic Advisory Board initiation ceremony and kick-off meeting
Conclude a Technology Development and Supply agreement with eZono AG (Germany) for Ultrasound-assisted needle guidance
Acquisition CuRAS tinea unguium onycomycosis insurance fee
Reticapture health insurance registration
Conclude a Collaborative Research and Development agreement with Markone Technology Co.,Ltd
KSCI (Korea Consumer Satisfaction Index) 1st prize
Received commendation as an Exemplary Taxpayer
Selected for P-TECH work-as-you-study program /
apprentice affiliate program
(Human Resources Development Service of Korea)
Secret RF ANVISA
FRAXIS PRO(Secret PRO) approval by Health CANADA
Designated as a supervising enterprise for the R&D Sandbox
Received award as a Leading Enterprise in Training Talented Professionals
Awarded Grand Prize in the SME Management Innovation contest
Obtained Innovative Medical Device certification
Selected for the (3rd) Designers Support project
Selected for Cross-Department Life Cycle Medical Device R&D project
Became listed on KOSDAQ
Selected for the Design Innovation Capacity Building Business Leaders program
Selected for the ICT Voucher Technology Development Business Leaders project
Selected as Strategic Responder to International IP Disputes
Selected as Global IP Startup
MAINBiz accreditation
Selected for work-as-you-study apprenticeship program / on-the-job training for students (Human Resources Development Service of Korea)
SECRET RF selected as a world-class product
Selected as an Excellent Company To Work In
Selected as Exemplary Tax-Paying Enterprise
Secret RF Smartcure Applicator FDA
Selected as a KB Best Enterprise
Opened Busan center
Received MDSAP system approval
Designated as 2019 Global Hidden Champion
Selected as SME Network Technology Development Business Leader
Selected for $10 million Export Tower award
Received confirmation as a leading enterprise in learning-centered on-the-job practical training [Sectors: electricity, electronics]
Selected as an Outstanding Employer (Gyeonggi Province)
Received accreditation in risk assessment (No. 18-22A-286)
Selected for Good Design (Tambor)
Selected for Good Design (Hyzer me)
Received participation award in 2018 Gyeonggi Regional Work-Life Balance
Outstanding Enterprise contest
Selected for Export Companies IP Convergence Development project
Selected for 2018 Gyeonggido Business & Science Accelerator
RetiCapture FDA
Selected as 2018 Outstanding Venture Business in the global category
Selected for joint technology development project between industry, academia and research institutes
CuRAS FDA
Appreciation plaque (Director of National Institute of Food and Drug Safety Evaluation)
Selected as Promising Outstanding SME (Small and Medium Business Administration)
FRAXIS FDA
Selected for joint technology development project between industry, academia and research institutes(Small and Medium Business Administration)
Selected for Seoul Asan Platform affiliate program(Ministry of Trade, Industry and Energy)
SECRET RF FDA
Obtained Hidden Champion certification(Minister of Employment and Labor)
Selected for 2017 ATC Excellent Technology Research Center project(Ministry of Trade, Industry and Energy)
Selected as 2017 recipient of promising ICT technology development support(Ministry of Science, ICT and Future Planning)
Opened Seoul and Daejeon centers
Selected as an Outstanding Employer (Gyeonggi Province)
Received 2016 Award of Merit for promoting venture businesses (Small and Medium Business Administration)
Prizewinner in November Pin Up Design Awards (Korea Association of Industrial Designers)
Selected as recipient of global strategic technology development support (Small and Medium Business Administration)
FRAXIS DUO FDA
Obtained certification as an Outstanding Enterprise for Compensating Employee Inventions (Korean Intellectual Property Office)
Obtained certification as an Outstanding Enterprise for Compensating
Employee Inventions (Korean Intellectual Property Office)
Capital increase without consideration
Selected as Outstanding Venture Business (Korea Venture Business Association)
Capital increase without consideration
Recapitalization (30 million KRW)
Selected as 2016 Global Hidden Champion (Small and Medium Business Administration)
Designated as AEO Generally Certified Outstanding Enterprise (Korea Customs Service)
Signed agreement for industry-academia cooperation with Kwangwoon University
Received commendation (Gyeonggi Regional SMEs and Startups Office)
Selected for work-as-you-study program / on-the-job training for students (Human Resources Development Service of Korea)
Joined the Women Friendly Enterprise Agreement (Paldal New Jobs for Women Center)
Selected as 2015 Outstanding Women’s Employment Enterprise by Gyeonggi Province
Signed agreement for improving the competitiveness of R&D platforms for medical devices based on regular ties between hospitals and companies(Seoul National University Dental Hospital/Seoul St. Mary's Dental Hospital/ilooda)
Selected as a 2015 Enterprise for Developing Technology to Reduce Social Gaps(Ministry of Science, ICT and Future Planning)
Csigned technology development agreement for line scan hair follicle removal laser surgery(Small and Medium Business Administration)
Selected for 2015 High Tech Medical Devices Development Support project(Ministry of Trade, Industry and Energy)
VIKINI FDA approval
Expansion and relocation of company office(120, Jangan-ro 458beon-gil, Jangan-gu, Suwon-si, Gyeonggi-do [Imok-dong])
Selected as a Top Enterprise by Kookmin Bank
Received achievement award from Ministry of Food and Drug Safety (CEO)
Signed agreement with Work to School Department at Eulji University
Received CEO letter of appreciation (KB Financial Group Inc)
Received accreditation in Korea Occupational Safety and Health Agency risk assessment
Selected for the 2014 Core Medical Devices Commercialization Support project(Ministry of Trade, Industry and Energy)
Signed cooperative MOU for the development of medical devices (Seoul St. Mary's Hospital)
Selected for the Export Capacity Building program in the field of promising export companies(Small and Medium Business Administration) Signed MOU for a specialized high school with Sun Moon University
Selected as 2014 recipient of support in obtaining overseas standards certification(Suwon Chamber of Commerce)
Signed agreement with the Work to School Department at Dongguk University
Received Commendation for Export Achievements from the Governor of Gyeonggi Province (CEO)
Selected for the $5 Million Export Tower award (Korea International Trade Association)
Signed industry-academia cooperation agreement with Suwon Technical High School
Selected as a 2013 Desirable Place to Work (Inno-Biz)
Selected as recipient of comparative clinical support by the Small and Medium Business Administration
Selected as an Outstanding Employer by Gyeonggi Province
Received participation award in open contest for export success stories (Korea International Trade Association)
Selected as a Promising SME by Kookmin Bank
Obtained KOTRA Global Brand certification
Selected as a 2013 Promising Company to Lead the Future (Korea News Today)
Signed operating agreement for a project to promote a specialized SME high school
Selected for Medical Device Translational Research Product Development project by Gyeonggi Province(high frequency temporomandibular joint treatment devices)
Selected as a Promising SME by Woori Bank
Signed MOU on industrial cooperation (Gumi Electronic Technical High School)
New CEO appointed
Selected as an enterprise to receive special military service exemptions
Selected as Promising SME (Gyeonggi Province)
Signed industry-academia agreement to support a Meister high school in the field of medical devices(Wonju Medical Instruments High School)
Received Regional Economic Development Award (Gyeonggi Province)
Selected as an Innovative Technology SME (INNO-BIS)
Selected for Small Business Innovation Research by the Small and Medium Business Administration(DPSS Green Laser)
ISO13485 certification
Recapitalization (300 million KRW)
Expansion and relocation of headquarters (High Tech Venture Valley in Gosaek-dong, Suwon)
Selected as Internet Trade Frontier Enterprise (Gyeonggi Province)
Obtained Venture Certification (Korea Technology Finance Corporation)
License obtained for manufacture of medical devices
Approval granted for attached research facility (Korea Institute for Advancement of Technology)
Registration of factory
Incorporation of ilooda Co., LTD
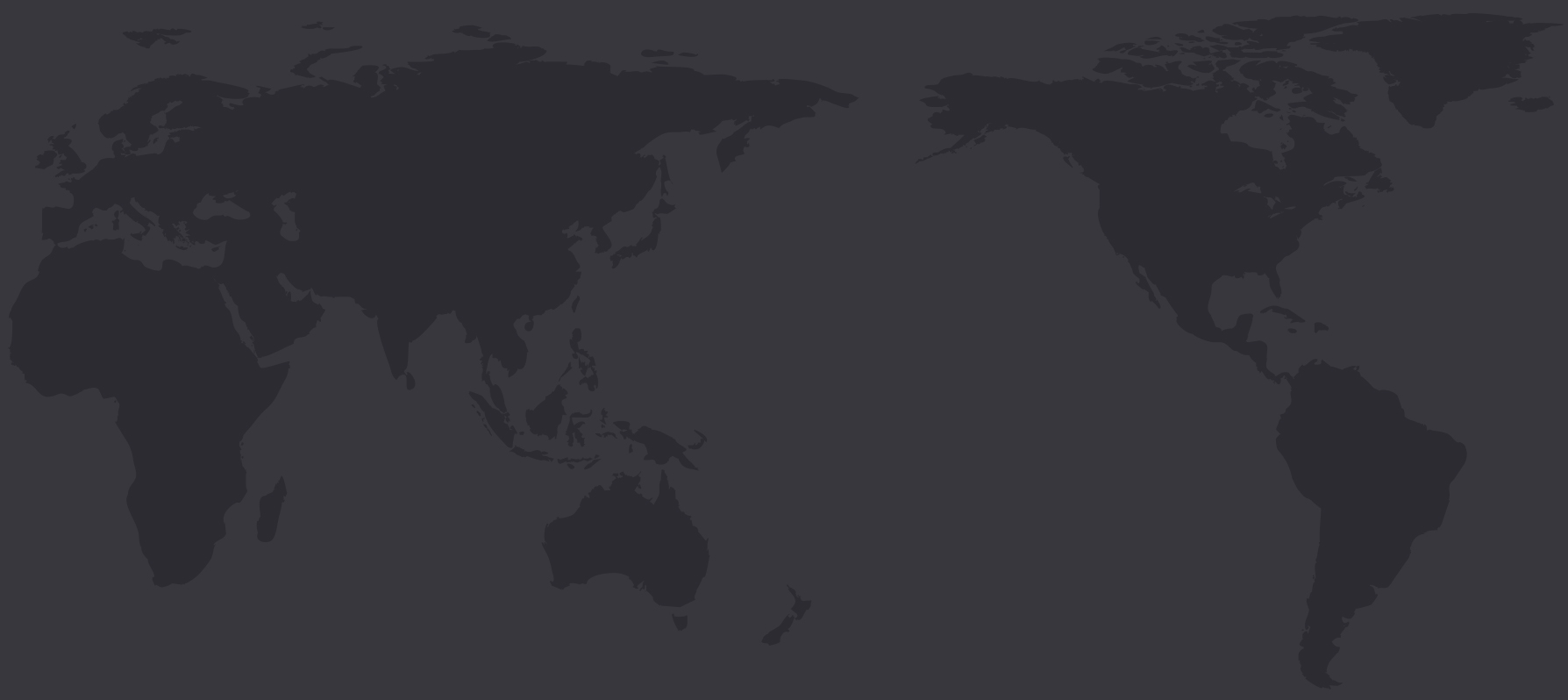
We have been recognized for our product and service excellence by prestigious experts in the medical industry from 50 countries worldwide
50 countries
Starting with US FDA registration in 2016, ilooda entered into the US medical equipment market and has been receiving great reviews.
Through sales of ilooda's proprietary brand, Secret RF, rather than an OEM or ODM brand in the US medical aesthetic market, we have proved our potential in the global market and continue to make a name for ourselves through product excellence.
 SECRET RF
SECRET RF
Through our wealth of clinical experience, we are focusing on developing innovative technology such as targeted lasers and AI image diagnosis for the near future.
We are also shoring up our communication and global partnerships with medical personnel for the development of new clinical ideas.

- All our products have the highest level of FDA approval compared to domestic competitors, and our professional medical devices are registered with the Ministry of Food and Drug Safety (six institutes)

- Pearl equipment infrastructure specialized for medical device development
- Leading domestic and overseas clinical trials and developing expertise in licensing
- High sales listing rate with a CAGR of 27.8% (2016-2019)
- Boosting product competitiveness by incorporating new technologies into standard products
- Product differentiation via the launch of innovative medical devices

- 67 distributors
in 52 countries around the world
- 26 Key Doctors
in 14 countries worldwide
- Global brand competitiveness
- 78.3% of all products exported in 2019

[Ministry of Health and Welfare]
Innovative medical device company certificate
2020-12-01

[Ministry of SMEs and Startups]
2020 Management Innovation Contest-Grand Prize
2020-11-25

[Public Procurement Service]
Innovative product designation certificate
2020-10-29

[Patent office]
Excellent company for job invention compensation
2020-06-26

[Ministry of SMEs and Startups]
Management innovation - small business confirmation
2020-03-18

[Ministry of SMEs and Startups]
Global aesthetic company
2019-04-23

[Korea Institute of Design Promotion]
Selected as an excellent industrial design product
2018-11-28

[Korea Industrial Designers Association]
Pinup Design Award Winner
2017

[Ministry of Industry and Trade]
Designation of Excellent Technology Research Center
2017-07-19
We can’t wait to show you
We are on a path that begins from the verge of a new era.
Get ready to transcend your limits with ilooda.